New electrolyte concept can enable safer lithium-metal batteries in high temperature
Lithium-metal batteries are useful for keeping grid stability because of their very high energy density. However, lithium-metal batteries are highly active and there is a need to make them safer. A new electrolyte concept has now been tested, with promising results, which can enable safer operations at high temperature. Quan Wu, PhD student at Chalmers, is working on the project “High temperature battery technology”.
Can you tell us a little about yourself, Quan?"I’m from China where I got my bachelor’s and master’s degree. In 2023 I applied for this PhD student position at Chalmers in Gothenburg. Actually, this was my first time going to another country. I really enjoy to see a different culture and I also find the weather pleasant here in Sweden. Where I’m from in China it’s very hot in the summer and then very cold in the winter. In my spare time I like to play badminton." |
 |
Why is there a need for high temperature batteries?
"Energy storage in general is crucial to keep the stability of the grid. However there are environments, like power-electronic converter halls, that have working temperatures with up to 50–80 degrees Celcius. The battery performance decay much faster in such an environment, and there are also safety concerns."
You are working with Lithium-metal batteries in this project. Why, and what is the difference compared to lithium-ion batteries?
"Lithium-ion batteries usually have a carbon or graphite compound in the anode to store the ions in the layer/pore structure. Lithium-metal batteries use lithium metal as the anode material, instead of carbon or graphite. The advantage with lithium-metal batteries is the much higher energy density, up to six times higher actually."
What are the problems related to lithium-metal batteries?
"Lithium-metal batteries that we have today are highly active and can be very dangerous, especially if exposed to air. There is also a problem with dendrite growth of the lithium metal. The dendrite growth can cause a connection between the positive and negative electrode and short-circuit the battery. What we want to achieve is to make the lithium-metal batteries safer so that we can take advantage of their superior energy density."
You have used ionic liquid as electrolyte.
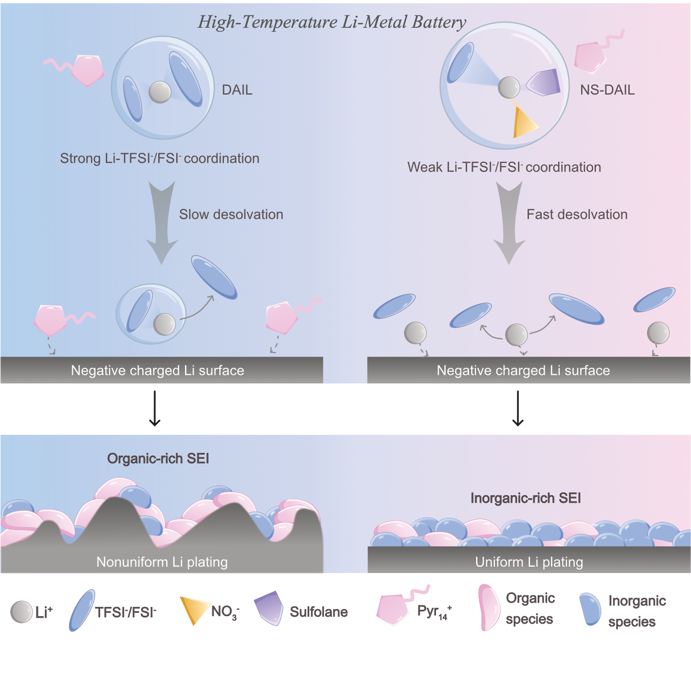
"Yes, as the electrolyte – the substance where the ions move between the cathode and anode – we have used an ionic liquid. The reason is that ionic liquid has a vapor pressure that is almost zero, which means that it will not evaporate even in high temperatures. Traditional organic solvent electrolytes dry out quickly when heated or exposed to air, and can lead to lost connection between the electrodes. Another advantage of ionic liquid is that it is almost fire proof, in comparison to traditional electrolytes which are fire-prone if exposed to heat or air, or encounter short-circuits. So, by using an ionic liquid the lithium-metal battery becomes much safer."
Are there any drawbacks with ionic liquid?
"Ionic liquid is very viscous and has a higher density, which hinders the ions’ movement in the electrolyte. To increase the ions’ mobility, without compromising with the safety advantages, we have added variations of lithium nitrate salt and sulfolane as electrolyte additives. This makes the anions – the negatively charged ions - more loosely coordinated to Li ions and therefore facilitates Li ions to transport more easily. The results from our tests have been successful, and shows that this electrolyte concept is promising."
How far is it to practical applications?
"Now we have focused on the electrolyte which is just one part of the battery. We still need to look at the positive and active electrode. Tests must also be conducted to ensure that the electrolyte functions properly in larger battery packs, and just not in a laboratory environment with small coin cells. So, practical applications is a bit ahead. But as I said this electrolyte is promising and it’s hopefully a step on the way towards a more efficient and safer high temperature battery."
When are you presenting the results?
"We have a paper under review that we hope will be published later this year."
Contact Quan Wu:
https://www.chalmers.se/en/persons/wuqu/
Partners in the project "High temperature battery technology"
Chalmers University of Technology, Hitachi Energy, Västra Götalandsregionen, Volvo Energy, Swedish Energy Agency
Project page